Healthcare logistics insights and news
Stay ahead of the curve with the latest insights in healthcare and pharmaceutical logistics. Discover the industry's toughest challenges and the smart solutions driving progress.
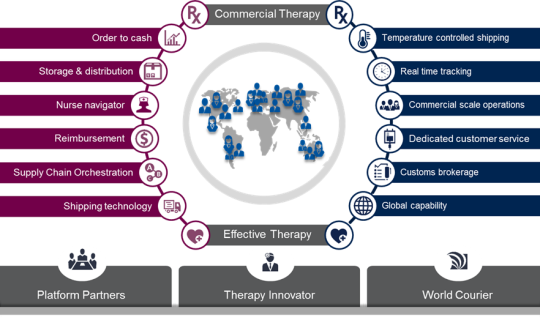
World Courier
Five signs you need a CGT logistics partner
January 2026





.png?h=320&iar=0&w=540&useCustomFunctions=1¢erCrop=1&hash=AF88FE243481C6E8DC2015CCC3FA512E)
.jpg?h=320&iar=0&w=540&useCustomFunctions=1¢erCrop=1&hash=07D76CBFF77F36BADCEFCBF11CB1A673)





































































.jpg?h=320&iar=0&w=540&useCustomFunctions=1¢erCrop=1&hash=31E00931176438BB94A402A98C4B5624)




.jpg?h=320&iar=0&w=540&useCustomFunctions=1¢erCrop=1&hash=46F1DAD400F81BCEBB3D04B0A8D757F2)









.jpg?h=320&iar=0&w=540&useCustomFunctions=1¢erCrop=1&hash=BEB1143FEB8A1E2AA74F394DAC9C2EC0)


.jpg?h=320&iar=0&w=540&useCustomFunctions=1¢erCrop=1&hash=FFAD46B50DC7BAF96943931DC619FE9D)

.jpg?h=320&iar=0&w=540&useCustomFunctions=1¢erCrop=1&hash=A24FECA9C9A36CF9E118BFB1F6855B5D)


































































































.jpg?h=56&iar=0&w=56&useCustomFunctions=1¢erCrop=1&hash=5C3BF081DEFE0CD4643E0D660948B3B2)


.jpg?h=320&iar=0&w=540&useCustomFunctions=1¢erCrop=1&hash=A0E2703FF178771B1EDD89EFC5F07029)









































.jpg?h=320&iar=0&w=540&useCustomFunctions=1¢erCrop=1&hash=DEA81FE4A2223DF4FABF912510B699F7)



























.jpg?h=320&iar=0&w=540&useCustomFunctions=1¢erCrop=1&hash=270DD3B1DF5FE745FD13ECA1BF898BFC)